Good pharmacology demands purity of the of the highest order. Quality is paramount. This is why, at Coral, we take immense pride in the quality of our products and go to great lengths to ensure that exacting standards are met l00% of the time.
Each of our processes has a validated SOP (Standard Operating Procedure) which we strictly follow. Audits, both internal and external, are regularly conducted to evaluate and maintain the health of our system. Our units, which comply with cGMP and GLP and are WHO and ISO certified, have been audited by the United States Food and Drug Administration (US FDA), the European Union (EU), other reputed regulatory bodies, and by our partners themselves.
Our people are our strongest asset, and our team is fully trained at induction. This is augmented by on-the-job training, which is followed by regular checks and validations to ensure adherence to policies and procedures.
Science does not tolerate inferior quality, and neither do we.
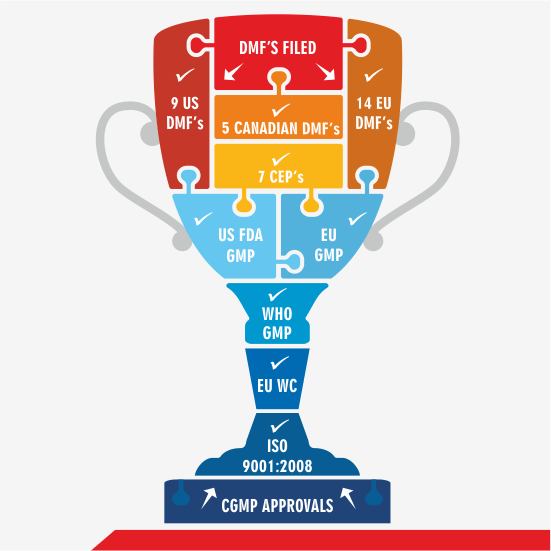

The partial list below demonstrates our commitments:
Approvals and Filings
- United States Food and Drug Administration (US FDA)
- European Union GMP (EU)
- Haryana FDA – GMP
- CDSCO – WHO GMP
- Written Confirmation as per EU Directive 2001/83/EC
- ISO 9001:2008 Quality Management Systems
- Drug Master Files (DMFs) and submissions worldwide
Commitment to Excellence
- Independent Quality Labs and Management Systems
- Independent R & D Labs
- In-house Microbiological Lab
- Validated process and systems
- Complete compliance to state industry norms

